In thermodynamics, the compressibility factor (Z), also known as the compression factor or the gas deviation factor, is a correction factor which describes the deviation of a real gas from ideal gas behavior behavior.
The calculations below can be used to find the inlet or the outlet compressibility factor by using the values for temperature and pressure. T and P are the temperature and pressure in units of °R and psia, respectively. The value calculated for Z should not be greater than zero. The compressibility factor has no units of measure.
Below are the steps and the equations to calculate the compressibility factor for a given gas based on the Redlich-Kwong equation of state:
Step 1: Calculate the Reduced temperature as:
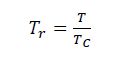
where T and Tc have the units of °R.
## Reduced temperature
## T and Tc have the units of °R.
def reduced_temperature(temperature, tc):
tr = temperature / tc
return tr
The reduced temperature of a fluid is its actual temperature, divided by its critical temperature. A critical point (or critical state) is the end point of a phase equilibrium curve. At the critical point, defined by a critical temperature Tc and a critical pressure Pc, phase boundaries vanish.
#python #programming #python3